EDTA Snakeoil! Ana Maria Mihalcea's Medical Malfeasance Exposed
“I already have had.. uh.. patients die from shedding” - Ana Maria Mihalcea, M.D.
“My people are destroyed for lack of knowledge: because thou hast rejected knowledge, I will also reject thee, that thou shalt be no priest to me: seeing thou hast forgotten the law of thy God, I will also forget thy children.” ~ Hosea 4:6 KJV
Rockefeller Medicine
Around 1900, the science world was getting excited about new “petrochemicals” and the ability to create a variety of new compounds from oil. Some of the first products derived from petrochemicals were plastics.
In 1908, the Rockefellers established modern medicine, which they dubbed “Allopathy.” The Rockefellers created the business of modern medicine, which has always been about poisoning people, Eustice Mullen explains.
The following is the definition of Allopathic medicine according to the NIH:
“A system in which medical doctors and other health care professionals (such as nurses, pharmacists, and therapists) treat symptoms and diseases using drugs, radiation, or surgery.”
The Rockefeller Institute for Medicine, founded in 1908, marked the advent of the re-creation of synthetic versions of natural cures. Before 1908, every place of healing in America, Europe, and the world used only ancient, traditional, natural medicinal cures. Every hospital was a “Homeopathic Hospital”. Most of these magnificent buildings were converted into mental health asylums where a system of torture and electric shock was established to “cure” mental illness.
John D. Rockefeller created the oil industry and used it to crush traditional medicine to enslave people. They also financed the Eugenics movement. One of the perks of modern medicine is depopulation.
“Everyone knows that the infamous Roe v. Wade opinion legalized abortion, but almost no one knows that legal abortion was a strategy by eugenicists, as early as 1939, to “genetically improve” the population by “reducing” it.”
“In the book, “Rockefeller Medicine Men: Medicine and Capitalism in America,” authored by E. Richard Brown, he tells the hidden story of the financial, political, and institutional manipulations whereby a diverse and eclectic range of traditional healing modalities available to the North American public was summarily canceled and pared down to a singular style of medicine that would become the predominant medicine of the Western world and a major force in global medical culture during the 20th century. This was brought about largely by the collaboration of the American Medical Association, the philanthropies of Andrew Carnegie and John D. Rockefeller, and the development of a revolutionary curriculum by the Johns Hopkins School of Medicine. Brown documents the story of how a powerful professional elite gained virtual hegemony in the Western theatre of healing by effectively taking control of the ethos and practice of Western medicine. E. Richard Brown describes how, in 1905, the American Medical Association’s new Council on Medical Education funded by Carnegie and Rockefeller commenced serious activity. They employed the services of Abraham Flexner, who proceeded to visit and “assess” every single medical school in the US and Canada. Within a short time of this development, medical schools all around the US began to collapse or consolidate. By 1910, 30 schools had merged, and 21 had closed their doors. Of the 166 medical schools operating in 1904, 133 had survived by 1910, and 104 by 1915. Fifteen years later, only 76 schools of medicine existed in the US, and they all followed the same curriculum.”
The 1910 Flexner Report laid the foundations of the modern medical system, dubbed “Rockefeller medicine” (Allopathy). Since 1910, corporate interests have established near total control of the medical field through pharmacology and their impact on medical education.
In 1935, vitamin C became the first artificially synthesized in Switzerland. Rockefeller saw a big opportunity in the possibility of developing vitamins and medications from petroleum. He saw the chance to control and monopolize multiple industries at once: petroleum, chemical, and medical. Petrochemicals were ideal from a business perspective because they could be patented, owned, and sold for high profits.
Today, the petrochemicals in plastics are causing a slew of illnesses, including neurodevelopmental disorders, diabetes, chronic respiratory disease, and cancer, which have increased between 28% and 150% between 1990 and 2019. Petrochemicals in microplastics are also rapidly reducing male fertility and polluting our environment. Please also read more here, here, and here.
The first pharmaceutical drug was an arsenic named Salvarsan. That’s right, an ARSENIC!
DEATH is an all-to-common side effect of pharmaceutical drugs, which is only logical when you administer poisons internally. The following pages demonstrate the many deaths of people around the world, most of them children, who were fatally poisoned during the first mass medication experiments with Rockefeller’s Allopathic Health. The paper entitled Toward Responsibility in International Health: Death following Treatment in Rockefeller Hookworm Campaigns, 1914–1934.
What is EDTA?
EDTA is synthesized on an industrial scale using 1, 2-diaminoethane (ethylene diamine), formaldehyde, water, and sodium cyanide.
Ethylene diamine induced acute and subchronic toxicity in lab animals, as well as allergic hypersensitivity. The liver and kidneys are target organs of ethylenediamine, where they stop working.
Read more: Is C60 And EDTA Safe? Clinical Review.
Absorption of large amounts of formaldehyde via any route can cause severe systemic toxicity, leading to metabolic acidosis, tissue and organ damage, and coma, according to the CDC.
Exposure to sodium cyanide can be rapidly fatal. According to the CDC, it has whole-body (systemic) effects, particularly affecting those organ systems most sensitive to low oxygen levels: the central nervous system (brain), the cardiovascular system (heart and blood vessels), and the pulmonary system (lungs).
EDTA is an industrial poison. The textile industry required a chelating agent to remove calcium during textile processing, and this led to the synthesis of polyamino-carboxylic acids, one of which was EDTA.
In 1935, a patent for industrial chemical use was filed for EDTA in Germany. EDTA is a synthetic acid effectively used to clean boiler rooms in nuclear power plants. In 1945, Franz Munz obtained a US EDTA patent. In 1947, the U.S. Food and Drug Administration (FDA) approved EDTA as a food additive in “low doses” because it’s a forever chemical and a preservative.
The U.S. FDA approves two different types of EDTA. In 1953, Edetate calcium disodium, also known as Calcium EDTA (marketed under the trade name Calcium Disodium Versenate registered ), was approved for treating lead poisoning. Three years later, in 1956, a related EDTA compound, Edetate disodium, was also approved for clinical use. This compound, also known as Disodium EDTA, has been marketed under the trade names Disotate (registered) and Endrate (registered). The essential difference between these two compounds is that Calcium EDTA’s structure has an incorporated Ca super(2+) moiety, while Disodium EDTA does not. The use of the latter compound, Disodium EDTA, has been associated with life-threatening and fatal hypocalcemia. Both EDTA are toxic.
While EDTA was approved for removing lead from the body, it has never been approved for removing other metals, as metals have different natures. Today, we have natural solutions and superior options for removing lead and other toxic metals from the body.
EDTA trial DEATHS
An EDTA trial (Sloth-Nielsen et al., 1981) on the possible antiatherogenic effect of EDTA with six patients showed clinical signs of potentially lethal hypocalcemia from abnormally low calcium levels caused by EDTA.
Another EDTA chelation human trial in 2003-2005 resulted in DEATHS due to hypocalcemia.
A 2006 EDTA chelation trial also resulted in DEATHS due to hypocalcemia.
There were several DEATHS reported from cardiac arrest due to lethal hypocalcemia in EDTA trials in 2006 and 2008 (Brown, Willis, Omalu, & Leiker, 2006; Baxter & Krenzelok, 2008), from calcium deficiency inducing alterations in the brain, and osteoporosis, which causes the bones to become brittle.
In 2008, a clinical trial with EDTA chelation on autistic children also proved fatal, resulting in DEATHS of children.
A 2007 EDTA chelation study proved KIDNEY FAILURE in humans.
Decades of clinical studies demonstrate that EDTA treatment is associated with severe, life-threatening adverse effects, as Science Direct explained in 2016.
“It should be emphasized that EDTA treatment is associated with severe, life-threatening adverse effects.
EDTA for cardiovascular disease DEBUNKED
Many Allopathic specialists tried to popularize the use of EDTA for chelation, to no avail. In the 1980s, Richard Casdorph, a practicing cardiologist, claimed improvements in heart ejection fractions and cerebral blood flow with EDTA chelation therapy in several articles. McDonagh, Rudolph, and Cheraskin published about 30 articles documenting various positive effects of EDTA chelation. This group wrote articles showing no problems with kidney function in patients treated with EDTA according to the published protocol. At the same time, conventional cardiologists wrote several editorials against EDTA chelation. So, the American Medical Association called for studies to see if chelation worked. The American Board of Chelation Therapy in 1983 was formed to certify doctors who give the therapy. It was later called the American Board of Clinical Metal Toxicology. ACAM also certified doctors who took its workshop on chelation therapy and passed its written and oral examinations. The Great Lakes College of Clinical Medicine later called the International College of Integrative Medicine (ICIM), was formed in 1983 to teach and research chelation and other integrative therapies. After complex negotiations, in the late 1980s, Walter Reed Army Hospital agreed to do a randomized clinical trial on EDTA chelation therapy. Partway through the study, it was suddenly discontinued for unknown reasons.
A systematic review of published articles in this field was undertaken in a paper by Seely, Wu, and Mills (2005). The authors concluded that the best currently available evidence did not support the therapeutic use of EDTA chelation therapy in treating cardiovascular disease. Similar results have been reported in review papers by Shrihari, Roy, Prabhakaran, and Reddy (2006) and Crisponi et al. (2015).
While a 2002 EDTA large randomized clinical trial “showed benefit,” smaller studies were inconsistent.
In the 1990s, the Federal Trade Commission filed a complaint against ACAM for claiming a brochure that chelation was effective for vascular disease. ACAM submitted almost 100 articles in support of the claim, but the FTC insisted that a large randomized trial was required to make that claim. ACAM finally gave up after spending a million dollars in legal fees and signed a consent order saying they would no longer make such a claim based on the evidence at that time.
“In conclusion, our study shows that in a population of stable, largely asymptomatic coronary artery disease patients with prior myocardial infarction, the use of EDTA chelation therapy did not produce a measurable change in health-related quality of life over 2 years of follow-up.”
A slew of other adverse events such as lacrimation, nasal congestion, mucocutaneous lesions, glycosuria, hypotension, and ECG abnormalities (DISEASE OF THE HEART AND LUNGS) have been reported as well as allergic reactions (Wax, 2013) to EDTA. Prolonged treatment with calcium EDTA leads to magnesium depletion and trace-metal depletion, the most marked being due to zinc excretion. Zinc depletion destroys your cells’ ability to absorb nutrients, leading to diabetes.
A 2015 study entitled, Quality of Life Outcomes with a Disodium EDTA Chelation Regimen for Coronary Disease: Results from the TACT Randomized Trial concluded with this statement:
“In conclusion, our study shows that in a population of stable, largely asymptomatic coronary artery disease patients with prior myocardial infarction, use of EDTA chelation therapy did not produce a measurable change in health-related quality of life over 2 years of follow-up.”
Severe kidney damage from EDTA chelation therapy was reported in a (Nissel 1986) trial. In a very short period, EDTA causes kidneys to shut down in complete failure.
A 2015 study suggests that EDTA chelation for myocardial infarction has “modest” benefits for cardio health. However, I would suggest that the moderate benefits of this study were due to the high doses of vitamin C that were also administered.
A 2017 study on EDTA chelation for atherosclerosis and Myocardial Infraction concluded:
“Unsubstantiated claims of chelation therapy as an effective treatment of atherosclerosis should be avoided and patients made aware of the inadequate evidence for efficacy and potential adverse effects, especially the harm that can occur if used as a substitute for proven therapies.”
Another 2018 EDTA trial concluded with this:
“These results… are not sufficient to support the routine use of chelation therapy for treatment of patients who have had an MI (Miocardial Infraction)”.
A 2023 study entitled Chelation Therapy Associated with Antioxidant Supplementation Can Decrease Oxidative Stress and Inflammation in Multiple Sclerosis: Preliminary Results proved a flop, with two participants discontinuing their trial participation.
EDTA for lead poisoning DEBUNKED
EDTA is touted as a treatment for lead poisoning. However, because of its adverse effects, calcium EDTA was replaced by DMSA in the treatment of lead poisoning (Aposhian et al., 1995) in 1995. CaEDTA has also been used for the treatment of cases with manganese toxicity, but the result was neurotoxic symptoms resembling PARKINSONISM (Andersen, 1999).
A 2004 trial showed that EDTA REDISTRIBUTES LEAD TO THE BRAIN after acute or chronic lead exposure (Andersen, 2004).
Another trial proved adverse effects in 5 patients receiving EDTA at an outpatient chelation clinic in 2002, and all patients experienced gastrointestinal and musculoskeletal symptoms.
Oral exposure to EDTA (2002) has produced adverse reproductive and developmental effects in animals. EDTA did not make it past the animal or human trials, so why are medical doctors using it in humans?
A 2002 EDTA trial was performed on humans as a test for “chelation” therapy by a chelation clinic, demonstrating adverse events in 5 out of 5 patients.
Additionally, EDTA is a persistent organic pollutant (POP). Each intake would only be partially excreted in that case, while the remaining chemicals build up in the body and produce cell death. Long-term exposure to calcium disodium EDTA creates toxicity and kidney damage.
EDTA Snakeoil Salesmen
In March 2023, Ana Maria Mihalcea interviewed Dr. Michael Roth, who claims that EDTA is a “synthetic amino acid related to vinegar.” Together, they make several medical claims that are not scientifically proven, such as that EDTA “detoxifies COVID vaccine, heavy metals, graphene oxide, parasites, hydrogels, and nanoparticles.”
The only scientific tool Ana Maria uses to back her claims is dark field microscopy. A dark field microscope cannot see nanoparticles; it takes a spectroscopy microscope to identify them. Her medical claims are fabricated and unscientific lies.
Ana Maria and Dr. Ross made additional unproven medical statements that “EDTA removes the effects of a heart attack, can bring back the elderly from senility and Alzheimer’s, reduces blood pressure, detoxifies several snake and spider venoms, lowers insulin, smooths skin and wrinkles, ” and a host of other laughable health claims not backed by quantitative scientific study.
Incidentally, Dr. Ross is now dead.
“Dr. Roth sadly passed away on March 11/2023”
My sources informed me that Dr. Rashid Buttar was using EDTA. Given that he was already severely poisoned, as Stew Peters reported, using EDTA Acid would have been enough to tip him over the edge and kill him.
EDTA is not an approved pharmaceutical drug. However, the FDA approved it for the COVID-19 emergency under Emergency Use Authorization (EUA), just like the modified RNA (modRNA) COVID-19 vaccine nanotechnology.
The National Center for Complementary and Integrative Health (.gov) clarifies that the FDA has not approved the use of EDTA chelation for heart disease.
Ana Maria has been touting EDTA as an “antioxidant” when it is not. She even published to her Substack that EDTA is an “Antioxidant” when it’s an acid poison and an oxidant. Many people saw it on her Substack.
I’ve had over a dozen clients come to me extremely sick from EDTA pills and EDTA IV infusion. Some clients thought it was a natural substance due to Ana Maria’s false advertising and medical malfeasance. A Medical Doctor is licensed to know whether EDTA Acid is an oxidant poison or an antioxidant. Not knowing this and inducing the death of a patient is not an acceptable excuse. Ana Maria is criminally liable.
EDTA is an oxidant when used internally. The studies that refer to EDTA as an “antioxidant” are in vitro lab studies, not in vivo (inside the body).
EDTA is used to preserve cell specimens for chemistry lab work because it prevents blood coagulation and oxidation of cells in a petri dish used for diagnostic purposes. This is the kind of “antioxidant” the studies are referring to. But when you infuse EDTA Acid into the human body, it acts as an oxidant poison.
EDTA also will not decoagulate the blood in vivo (inside the body). But I can see how people who cannot read peer-reviewed literature could be deceived and manipulated by snake oil salespeople.
For example, a study entitled “Comparative study of the antioxidant capability of EDTA and Irganox.” EDTA is a preservative used in laboratories to preserve cells for scientific lab research. EDTA prevents the oxidation of cells in a petri dish. Oxygen causes cells to deteriorate, but labs need them to last longer for clinical research. It’s a different story when used inside the human body (in vivo). Then EDTA acts as an oxidant poison, not an antioxidant. So, this has a very different meaning.
One of the well-documented and widely known adverse events from EDTA “chelation” is DEATH, according to Mount Sinai.
“Other serious side effects that have been reported include low blood sugar, diminished calcium levels, headache, nausea, dangerously low blood pressure, kidney failure, organ damage, irregular heartbeat, seizures, or even death.”
I have to wonder if Ana Maria Mihalcea is informing her patients that death is a potential adverse event to EDTA chelation. In a March 24, 2024, broadcast that Ana Maria released, at the 53:24 minute mark, she makes a chilling confession:
“I already have had… uh… patients die from the shedding.”
How many of Ana Maria Mihalcea’s patients have been killed by her EDTA infusion protocol? I was horrified when I heard Ana Maria’s confession because I haven’t had any clients die from shedding! I’ve treated many people who were extremely sick from shedding, and I helped them all to detox effectively. Some clients came to me after several hospitalizations from extreme shedding, but none of them died in my care! Nobody needs to die from shedding if they use an effective detox protocol.
For 3 years now, Ana Maria has claimed that EDTA detoxes graphene, dissolves graphene, and chelates heavy metals, but experts such as Dr. Robert Young and Dr. Judy Mikovitz told me this is impossible.
Ana Maria has even produced a medical study with unscientific claims right in the title, “EDTA Chelation Dissolves the Artificial Intelligence Magnetic Hydrogel Weapon.” Health Canada also promoted the study. In her study, Ana Maria claims that EDTA can “detoxify the body even from Graphene.”
EDTA is not a detox agent! Again, it’s an oxidant that degrades cells, whereas genuine antioxidants repair cells.
Ana Maria does know about oxidant poisons. In an interview from December 2022, she referred to graphene as an oxidant. At least she’s correct about something.
Saul Green, Ph.D., and Wallace I. Sampson, M.D. wrote in great detail about the Implausibility of EDTA Chelation Therapy, stating:
“EDTA chelation effectiveness is implausible; (2) the preponderance of evidence shows ineffectiveness; and (3) EDTA augments oxidative reactions involving iron instead of inhibiting them, resulting in increased likelihood of production of oxygen free radicals rather than neutralization of them, as claimed.”
EDTA is a precursor to cellular transfection
The Rockefeller Institute of Medicine has done clinical research on EDTA. One study, Studies of Cell Deformity, from 1967, shows that cells will degrade from EDTA exposure, which also induces “deformation” on their surfaces. The trial demonstrated that EDTA stops the cellular synthesis of calcium. They learned that calcium is bound to anionic sites at the cell periphery, some of which are located at the cellular electrokinetic surface.
Due to Rockefeller’s research, EDTA is now used in electrophoresis, a laboratory technique for separating DNA, RNA, or protein molecules based on their size and electrical charge. According to the National Human Genome Research Institute, an electric current moves the molecules through a gel or other matrix.
In agarose gel electrophoresis, EDTA is added to chelate the magnesium ions, cofactors for DNA nucleases. Hence, the activity of DNA nucleases that may be present is inhibited, and “DNA is protected from degrading.” EDTA is an effective transfection agent because it dissolves parts of your DNA, preserving cells for lab research in vitro.
Gel electrophoresis using EDTA is routinely used for the detection and size analysis of proteins and nucleic acid. DMSO is used with EDTA in this process. This destruction of cells makes transfection (gene editing) of cells easier using CRISPR-Cas9, which splices and dices the genome in vivo, as this study explains, “Inhibition of CRISPR-Cas9 ribonucleoprotein complex assembly by anti-CRISPR AcrIIC2.”
EDTA was found to be genotoxic in laboratory animals. A study from 1983 demonstrates that EDTA induces gene mutations and chromosomal breakage, meaning that genetic mutations will be passed on to your offspring, affecting generations to come, according to this Genetic Toxicology of EDTA study from 1983.
Calcium chelate of EDTA (CaEDTA) “chelation” has shown teratogenic effects (Catsch & Harmuth-Hoene, 1976), which are central nervous system depression and peripheral neuropathy. EDTA produced abnormalities in pups of rats removed by a cesarian section on day 21 of the study. Increases in several abnormalities (cleft palate, adactyly or syndactyly, abnormal rib or abnormal vertebrae) were observed with increased doses of CaEDTA.
According to the NIH, EDTA improves the transfection of embryonic stem cell lines (hESC) in cells.
According to a peer-reviewed paper by the NIH, EDTA is a precursor to cellular transfection.
“We found that chemically abrading the differentiated CACO-2 human intestinal epithelial cell layer by a trypsin and EDTA pretreatment (before the use of detergent-like transfection reagents) dramatically improved transfection efficiency in this polar, differentiated model. Although this treatment did improve the transfection efficiency, it also induced leakiness in the epithelial barrier by both opening tight junctional complexes and by creating holes in the cell layer because of low-level cell death and detachment. Thus, this approach to enhance the transfection efficiency of polar, differentiated cells will be useful for assessment of the effect of the transfected/expressed protein on (re)formation of an epithelial barrier..."
Thermo Fisher Scientific Inc. Fetal Bovine Serum for human transfection also uses EDTA.
An NIH study entitled “Kinetic Basis for DNA Target Specificity of CRISPR-Cas12a” reveals that EDTA enables rapid binding to DNA during gene editing (transfection).
Another study entitled “Improved genome editing by an engineered CRISPR-Cas12a” explains:
“CAS12a is an RNA-guided, programmable genome editing enzyme found within bacterial adaptive immune pathways. Unlike CRISPR-Cas9, Cas12a uses only a single catalytic site to both cleave target double-stranded DNA (dsDNA) (cis-activity) and indiscriminately degrade single-stranded DNA (ssDNA) (trans-activity).”
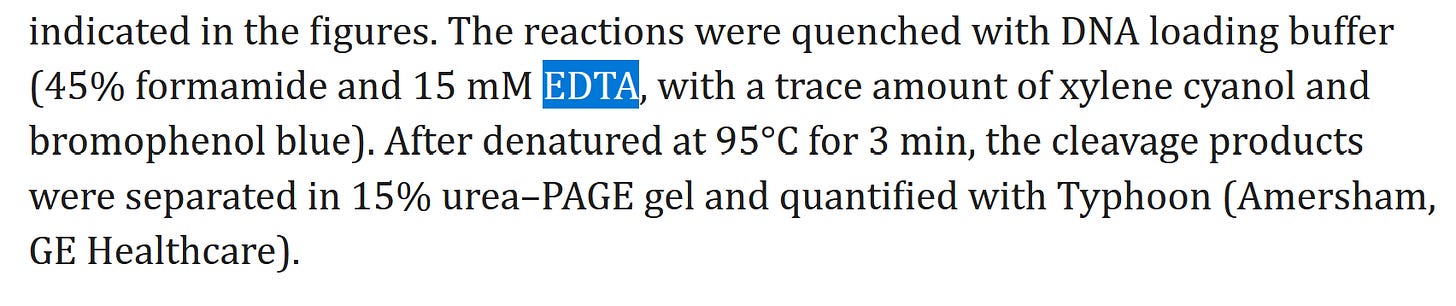
According to the study, “A DNA loading buffer of 45% formamide and 15 mM EDTA, with a trace amount of xylene cyanol and bromophenol blue…” is used to transfect the human genome.
EDTA is a transfection agent used with CRISPR-Cas9 to edit the human genome. Does EDTA “dissolve” graphene, as Ana Maria claims? The answer is NO! EDTA reduces graphene to a Reduced Graphene Oxide (RGO) form. Graphene is reduced by oxidation. Rather than dissolving graphene, EDTA reduces it to Graphene Oxide Nanoparticles.
Graphene oxide is more toxic than graphene, as I documented in my article “Graphene Oxide: The Vector For COVID-19 Democide.”
I will emphasize again that Graphene Oxide Quantum Dots cannot be seen with a dark-field microscope. So Ana Maria’s claims that EDTA detoxes graphene from the human body are unscientific.
EDTA chelation is NOT effective in removing metals from the human body. It’s a different kind of “chelation” being referred to when used to create electrochemical sensors (biosensors) with graphene!
EDTA serves as a connecting mediator between NiHCF (Graphene Oxide Nanoparticles and Nickel) and graphene nanosheets. EDTA is used with metal nanoparticles, metal oxides, graphene, carbon nanotubes, and quantum dots to stabilize the technology for a more uniform distribution throughout the body.
A Science Direct paper entitled “Highly sensitive ascorbid acid sensors from EDTA chelation derived nickel hexacyanoferrate/graphene nanocomposites” reveals that EDTA is used to create Graphene/Nickel, AA sensor nanocomposites.
“EDTA chelation stragey” is used for the “homogeneously distrubuted” NiHCF” (Nickel hexacyanoferrate composite) on graphene sheets. EDTA residue-supported pyramidal and spherical nanoparticles of NiHCF deposited on graphene sheets are used to create biosensors for forming Graphene/Nickel hydrogels.
The graphene hydrogel nanocomposite sensors (Gr/NiHCF) are used as externally controlled biosensing tools. They tell us they’re used to test for ascorbic acid, but this technology is also used for “human life” and industrial use. See the link here.
Graphene oxide/Lauric acid nanoparticles are modified using EDTA. Lauric acid nanoparticles are suggested as a “prospective drug carrier” for oral nanoparticle-mediated sustained drug delivery (timed release technology) used for the removal of Pb(II) ions (lead). However, studies show there are cytotoxic results.
Another study, “Improved genome editing by an engineered CRISPR-Cas12a,” demonstrates how EDTA improves transfection and modification of the human genome.
Once Graphene is reduced to Graphene Oxide, due to its small particulate size, you can no longer identify it using a Dark Field Microscope. Ana Maria uses a dark field scope, not a spectroscopy microscope. Spectroscopy is the only microscope that can measure GON and Quantum Dots.
So, what is Ana Maria doing with EDTA infusions? She’s creating a metal-EDTA complex that stabilizes and strengthens the graphene-based nanotech weapon system and enables it to spread more readily throughout the body for human transfection. As she reveals in an interview, she uses “light and sound healing techniques” to activate the delayed release technology before administering EDTA infusion.
EDTA is a poisonous acid that dissolves DNA. It’s used to prime the cells’ DNA for transfection. EDTA disrupts the surface of skin cells so that other chemicals can penetrate more easily, and CRISPR-Cas9 gene editing technology can work more efficiently.
The NIH describes EDTA’s enhanced cellular transfection:
“Flow cytometric analysis using an enhanced green fluorescent protein vector showed a significantly increased transfection efficiency of EDTA method compared to standard enzyme method. In addition, the EDTA approach maintained stable cell viability and recovery rate of hESCs after transfection.”
Another study published in Research Gate confirms that EDTA increases cellular transfection, along with using chloroquine.
Graphene Oxide Quantum Dots (GOQD-HA) nanocomposite uses EDTA for tissue-specific delivery of Metformin, an anti-diabetic drug known as insulin.
Conclusion
Beware of snake oil salesmen, and never trust pharmaceuticals. Superior heavy metal chelation supplements exist, such as ASEA redox molecules and Master Peace. Sign up and order here.
Medicines made from nature are always superior to pharmaceutical drugs. Finally, be sure your Naturopathic Doctor is competent!
Schedule a health consultation with me for a customized health protocol.













There has been confusion in the past over different forms of EDTA.
From https://sciencing.com/what-is-edta-5002665.html "Some of the toxic effects of EDTA have resulted from human error. It is easy to confuse the calcium-containing (calcium disodium EDTA) and non-calcium-containing forms of EDTA (edetate disodium).
When edetate disodium was mistakenly administered to patients, it caused rapid hypocalcemia that proved fatal. For this reason, edetate sodium is no longer commercially available in the US. Calcium disodium EDTA hasn’t caused any reported deaths from hypocalcemia."
Yandex search: https://yandex.com/search/?text=confuse+the+calcium-containing+(calcium+disodium+EDTA)+and+non-calcium-containing+forms+of+EDTA+(edetate+disodium).&lr=10636
I always thought Ana was off and couldn't possibly be writing all that herself and when she made the post with snakes going thru a free mason checkerboard over a pyramid with talk about interdimensional beings along with Annunaki and fallen Angels, and gave a few paragraphs ingratiating Christians I knew 0% of what she said could be trusted and was a 3-letter Agency agent. I had written these things in comments many times and I mainly got the vibe I was a party pooper.